As explained by Katherine Eban, in her excellent book "Dangerous Doses", the problem of counterfeit pharmaceuticals has grown from a few unscrupulous PBMs (pharmacy benefit managers) buying the stock of bankrupt Pharmacies on the gray market, and reselling it as if it came directly from the manufacturer, to today where criminal gangs are introducing counterfeit and fake pharmaceuticals into the supply chain.
Today, many pharmaceuticals have long supply chains, with many generics being manufactured in China, and passing through many hands before being dispensed or administered in the USA. Also, some pharmaceuticals are very expensive, making counterfeiting much more profitable for criminal enterprises than dealing in illegal "street" drugs such as heroin or fentanyl.
It is the responsibility of organizations, such as repackagers and dispensers of pharmaceuticals, to try to prevent the use of both gray market and counterfeit pharmaceuticals.
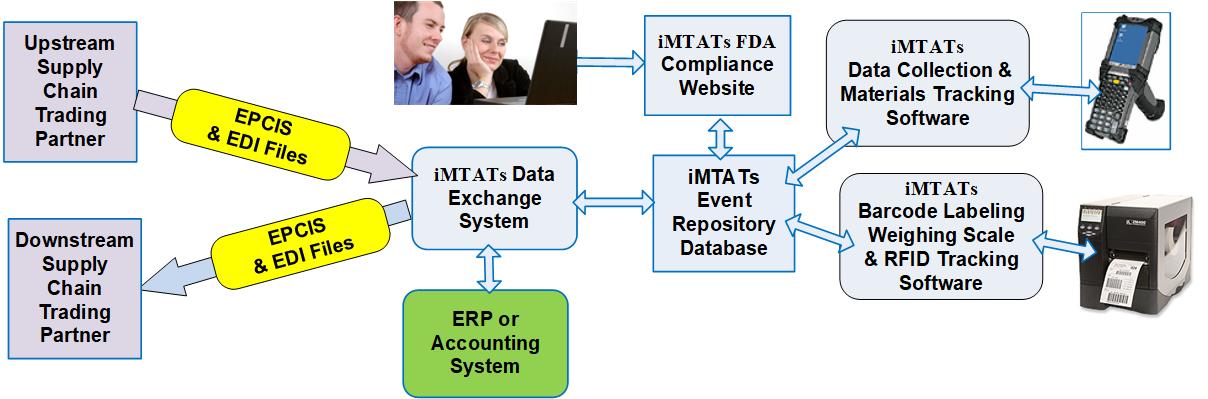
In this white paper, we explore how systems such as the intelligent Materials Tracking and Traceability software (iMTATs) can be used to detect and prevent counterfeit pharmaceuticals from being used.
For more details, please click on
Validating Pharmaceuticals Received from Supply Chain Trading Partners
| Features | Software |

|
Architecture | Background |
| Benefits | White Papers | FDA/GS1 Compliance | Videos | Partner Info |
| Software |

|
Architecture |
| Background | FDA/GS1 Compliance | White Papers |
| Partner Info | ||
| Features | Software Prices | Benefits |
Copyright © 2024 Smart Operations Management LLC