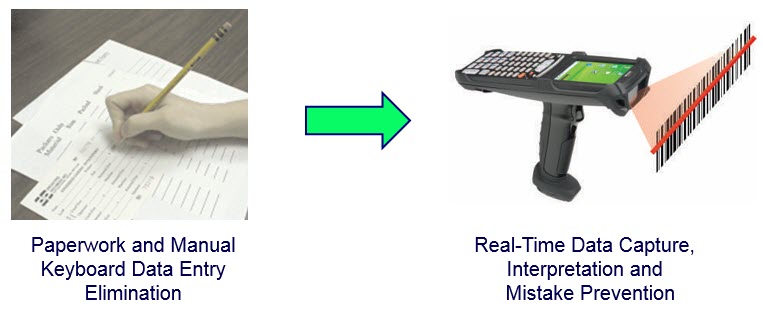
Keeping track of all the required materials tracking and traceability data, required for FDA compliance, using paper forms and Excel spreadsheets can be an overwhelming task requiring the time of many people who, otherwise, would be doing productive work. That is not to say that this is not important work, just that much of it can be automated.
We have seen examples where as many as the time of ten full-time equivalent (FTE) people, at a cost of over $70,000/year per FTE , could be saved by automating the capture of materials tracking data, the printing of GS1 compliant barcode labels, and the exchange of data with supply-chain trading partners.
Some of the ways that iMTATs achieves this savings are:
There is no per user, per customer, per supplier, or per end point/system, or per transaction licensing fee for iMTATs data exchange, provided that it is run on the client's own computers or computers that the client directly rents from a third party.
As such, iMTATs offers as alternative to Cloud-based FDA compliance services, especially those for FSMA and DSCSA compliance, which charge a per user, per customer, per supplier, or per end point/system, or per transaction usage fee, which, while convenient, can be very expensive for those organizations with many customers or suppliers or a high transaction volume.
The iMTATs software is intended to be implemented by organizations with their own IT departments, or by systems integrators who are Milramco partners and implement FDA compliance systems for their clients. As such, iMATs is designed to be readily customized by the IT staff to meet the requirements of each individual enterprise or for different departments within the Enterprise.
iMTATs may also be offered by ERP resellers and service bureaus who are Milramco licensees, and offer iMTATs as a part of their services to their clients.
| Features | Software |

|
Architecture | Background |
| Benefits | White Papers | FDA/GS1 Compliance | Videos | Partner Info |
| Software |

|
Architecture |
| Background | FDA/GS1 Compliance | White Papers |
| Partner Info | ||
| Features | Software Prices | Benefits |
Copyright © 2024 Smart Operations Management LLC