Drug Supply Chain Safety Act (DSCSA) Applications of the
intelligent
Materials Tracking and Traceability Software (iMTATs)

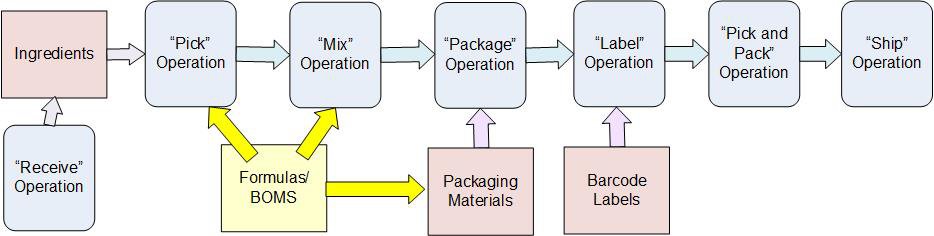
The intelligent Materials Tracking and Traceability software (iMTATs) is an affordable CFR 21 Part 11 compliant system that can be
used to track the manufacture, repackaging, and distribution of pharmaceutical
products.
iMTATs meets the requirements of the Drug Supply Chain Safety Act (DSCSA)
in the following ways:
- Printing out unique GS1 compliant 2D Electronic Product Code (EPC)
barcodes, containing GTIN/NDC product codes, as well as unique serial
numbers, plus lot numbers and expiration dates. These can be printed on
demand or in preprinted rolls, to apply to bottles and other containers
of pharmaceuticals, to uniquely identify each container in the global
supply chain.
- Printing out unique GS1 compliant Serialized Shipping Container Code
(SSCC) barcodes to apply to shipping cartons, pallets and other logistic
containers. These can be printed on demand or in preprinted rolls, to
apply to shipping containers, such as cartons and pallets as well as
other logistic containers, to uniquely identify each logistic container
in the global supply chain.
- Capturing Drug Supply Chain Safety Act (DSCSA) event history data
using barcode or RFID scanning. This includes origination, receiving,
movement, transformation/manufacturing, packing and shipping events as
well as the destruction or adjustment of inventory. This includes
automatically decoding the 2D barcodes on containers of pharmaceuticals
to extract the manufacturer, their NDC code, the GTIN (Global Trade
Identification Number), the lot and serial numbers as well as the
expiration date.
- Using the captured event data to provide point-of-action warnings if
the person doing the real-time data entry is about to make an
operational mistake, such as using materials that have not passed
quality control inspection, or is about to make a data entry mistake,
which could impact traceability.
- Performing backwards and forwards traceability, based on the
captured event data, to assist in determining the source of defects and
the containers of material impacted by a defect. This includes
determining to which downstream supply chain partners potentially
defective materials were shipped to so that these materials can be
quickly and easily recalled.
- Automatically receiving EPCIS files containing DSCSA Event tracking
data from upstream supply chain partners and integrating this with
locally generated event tracking data.
- Automatically sending GS1 Compliant EPCIS files containing event
history data to downstream supply chain partners so that they can
integrate this with locally captured Drug Supply Chain Safety Act
(DSCSA) event data.
- Providing a web portal through which downstream supply chain
partners, as well as Drug Supply Chain Safety Act (DSCSA) compliance
regulators, such as from the FDA and State public health authorities,
can obtain the electronic pedigree of a specific container of
pharmaceuticals or a batch of products in a specific shipment.
Please click here to learn more about
Using iMTATs for Materials Tracking and Traceability.